*Alnylam is proud to feature real patients in our advertising. Patients may or may not be on an Alnylam therapy.
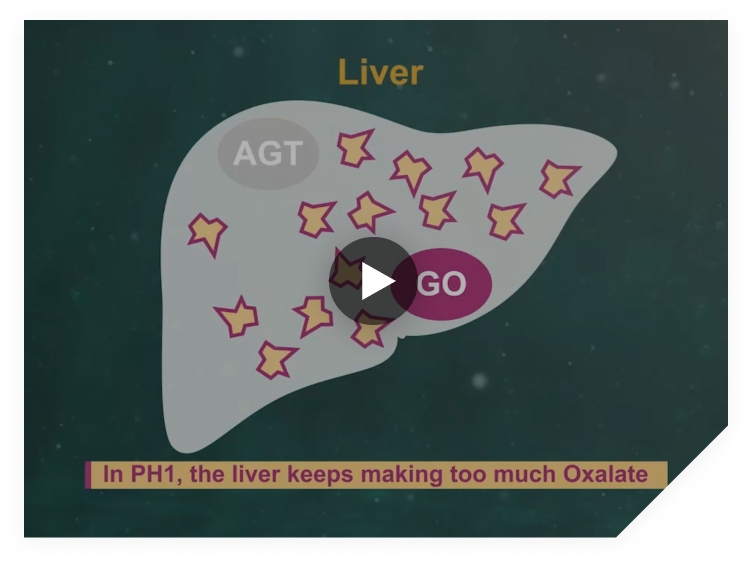
OXLUMO works by reducing oxalate production
OXLUMO is the first approved treatment for infants, children, and adults with primary hyperoxaluria type 1 (PH1).
OXLUMO is a prescription medicine for the treatment of PH1 to lower oxalate in urine and blood in children and adults.

CONNECT WITH AN ALNYLAM EDUCATOR
Learn about primary hyperoxaluria type 1 (PH1) and treatment with OXLUMO® from one of our trained Alnylam Patient Education Liaisons (PELs).
PELs are employees of Alnylam Pharmaceuticals. They are not acting as healthcare providers and are not part of your healthcare team.
How OXLUMO was tested in adults and children 6 years and older
OXLUMO was studied in the largest clinical trial of patients with PH1 ever done. It was a 6-month study with 39 adults and children 6 years and older who were diagnosed with PH1, did not have advanced kidney disease, and were not on hemodialysis. During the study, 26 patients received treatment with OXLUMO, and 13 patients received a placebo (an injection containing no medication).
After 6 months, those initially on placebo were switched to OXLUMO, while patients who were receiving OXLUMO continued receiving OXLUMO.
Because urine is the main way oxalate is removed by the kidneys, the trial looked at the amount of oxalate in urine.
Results after 6 months:
After 6 months of treatment, patients on OXLUMO had on average 53% less oxalate in their urine than patients on placebo
- Patients initially on placebo who switched to OXLUMO had similar reductions in urinary oxalate after 6 months of treatment
Results after 30+ months:
of patients on OXLUMO had normal, or close-to-normal, oxalate levels* in their urine after 36 months.
of patients who switched to OXLUMO from placebo had close-to-normal oxalate levels* in their urine after 30 months on treatment.
A normal level of oxalate in the urine means that oxalate levels were no longer elevated above the normal range. A close-to-normal level of oxalate in the urine means that oxalate levels were elevated but within 1.5 times the higher end of the normal range.
How OXLUMO was tested in infants and children younger than 6 years
In another clinical trial, OXLUMO was studied in 18 patients (infants and children younger than 6 years old) diagnosed with PH1, who did not have advanced kidney disease, and were not on hemodialysis:
- All patients in the study received treatment with OXLUMO for the initial 6 months, then continued receiving OXLUMO
Because urine is the main way oxalate is removed by the kidneys, the trial looked at the amount of oxalate in urine.
On average, patients treated with OXLUMO had 72% less oxalate in their urine† after 6 months of treatment, and 76% less oxalate in their urine† after 30 months of treatment, compared to the start of the study.
Measured by the ratio of oxalate in the urine to creatinine level.
OXLUMO was also tested in patients with advanced disease, including people on hemodialysis
In an additional clinical trial, OXLUMO was studied in 21 patients of all ages (including infants younger than 1 year old) diagnosed with PH1 who had advanced kidney disease. The patients were divided into 2 groups:
- Group A was made up of 6 patients who were not on hemodialysis
- Group B was made up of 15 patients who were on hemodialysis
All patients in the study received treatment with OXLUMO for the initial 6 months, then continued receiving OXLUMO
The trial looked at the amount of oxalate in blood, which is where it collects in patients who have advanced kidney disease.
After 24 months of treatment with OXLUMO
OXLUMO was effective in reducing levels of oxalate in urine in infants, children, and adults, as well as reducing oxalate in blood in patients with advanced kidney disease.
Safety profile of OXLUMO
Clinical trials evaluated the safety profile of OXLUMO in 98 patients with PH1. Patients were 4 months to 61 years old at first dose.
The most common side effect in OXLUMO clinical trials was injection site reaction. Symptoms included redness, swelling, pain, bruising, itching, and discoloration at the site of the injection. Symptoms were generally mild, resolved within one day of injection, and did not result in stopping treatment.
- OXLUMO: 38%
- Placebo: 0%
- OXLUMO: 15%
- Placebo: 8%
After 36 months of treatment, the side effects of OXLUMO were consistent with the first 6 months of the study.
In the studies evaluating infants and children younger than 6 years (up to 30 months), and patients with advanced kidney disease including those on hemodialysis (up to 24 months), the side effects of OXLUMO seen in these studies were similar to the study above.
Each patient will respond differently to treatment with OXLUMO. Talk to your doctor about any and all side effects you experience.
For more information about the potential side effects of OXLUMO, talk to your doctor.
You can also read the Prescribing Information.
We're here to help you access therapy
Find support services that can help you and your family during treatment with OXLUMO.